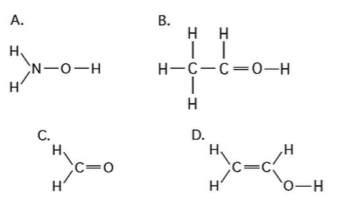
Which of the Following Molecules Is Polar C3h7oh C2h5cooh
D C2H5COOH is not polar but C3H7OH is polar. Which of the following molecules is polar.
Chapter 4 Flashcards Easy Notecards
Which of the following molecules is polar.

. C3H7OH and C2H5COOH are both polar molecules. Is C2H5COOH organic or inorganic. 3C3H7OH and C2H5COOH both are polar molecules.
Which of the following is. Evaporated water conversion to solid water molecules. Question 14 Which of the following is one reason that African slaves were sent to the Americas.
Which of the following can carbon-based molecules do because of the versatile bond structures formed by carbon. C3H7OH is not polar but C3H7OH is polar. C3H7OH and C2H5COOH are both polar molecules.
Calculate the pH of the solution and the concentrations of C2H5COOH and C2H5COO- in a 00671 M propanoic acid solution at equilibrium. C3H7OH is not polar but C3H7OH is polar. ExplanationIn winters the cold air masses move from cold areas to warm lakesAbove warm lakes there are water vaporsThis vapore converted to snow crystals from the cold air massesThen these solid water molecules will return to earth in.
C2H5COOH is 134 x10-5. Which of the functional groups below acts most like an acid in water. Up to 256 cash back Get the detailed answer.
Which of the following molecules is polar. Which of the following molecules is polar. O Neither C2HsCOOH or C3HOH is polar.
A compound contains hydroxyl groups as its predominant functional group. Are c3h7oh and c2h5cooh polar molecules. C3H7OH and C2H5COOH are both polar molecules.
A compound contains hydroxyl groups as its predominant functional group. There was a need for a cheap labor force to. Which two functional groups are always found in amino acids.
A compound contains hydroxyl groups as its predominant functional group. View the full answer. Thalidomide and L-dopa shown below are examples of pharmaceutical drugs that occur as enantiomers or molecules that _____.
Have mirror-image versions. Make three-dimensional shapes b. Do you know the correct answer.
If we supply heat to break these hydrogen bonds then the molecules goes into gaseous vapor phase. Amino acids are acids because they always possess which functional group. Which of the functional groups below acts most like an acid in water.
A 31 Which of the functional groups is not reactive but serves as a recognizable tag on the DNA molecule and alter the expression of genes in the cells. She also weighs 5000 g of DI water. С2H5COOH is not polar but C3H7OH is polar O C2HsCO0H is polar but CsHyOH is not polar.
The Ka of propanoic acid C 2 H 5 COOH. Neither C2H5COOH or C3H7OH is polar. Are mirror images of one another.
Which of the following molecules is polar. Which of the following molecules is polar. They are both polar molecules.
C2H5COOH is polar but C3H7OH is not polar. Questions in other subjects. Should dissolve in water.
C3H7OH is an alcohol which has polar groups hydroxyl group and C2H5COOH is a carboxylic acid which also has a polar groupcarboxylic group so bith are polar but polarity of carboxylic acid is greater. Therefore this compound _____. Are c3h7oh and c2h5cooh polar molecules.
C3H7OH and C2H5COOH are both polar molecules. 3C3H7OH and C2H5COOH both are polar molecules. If we supply heat to break these hydrogen bonds then the molecules goes into gaseous vapor phase.
See answer 1 Best Answer. C3H7OH and C2H5COOH both are polar molecules because o. They are both polar molecules.
Which of the following molecules is polar. O C3HyOH and C2HsCOOH are both polar molecules. Kemmi Major weights 1530 g of ammonium chloride NH4Cl salt.
Which of the following molecules is polar. You know the right answer. Water molecules in liquid form are attracted to each other by hydrogen bond that prevents them from transforming to gaseous form by acquiring kinetic energy.

Biology Review Chapter 4 6 Flashcards Quizlet

Comments
Post a Comment